This post was originally published in Current Science Review on January 27, 2015 and can be found here.
Pathology: Origins
Rudolf Virchow touted nothing short of an impressive resume: anthropologist, prehistorian, biologist, writer, editor, politician, public health expert, even “The Pope of Medicine” (though as a strong opponent of evolution, Virchow just misses mark for paragon of progress) (1). Perhaps most notable of Virchow’s titles is the “Father of Modern Pathology”. In 1858, Virchow began using microscopes in medicine to inspect human tissue. His work played a major role in eradicating ideas of humourism, which garnered surprising credibility even until his time (2).
Rudolf Virchow pioneered the field today that uses stained biopsies to diagnose disease, determine disease state, and recommend treatments. Pathology has matured greatly over the past 150 years. Though, since Virchow introduced the microscope to medicine, pathology has yet to experience a paradigm shift—no substantial changes or groundbreaking technologies that issue multi-fold improvements on the state of the art the way that Virchow did in 1858.
A Digital Approach
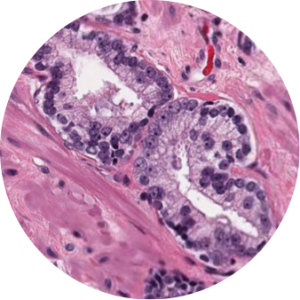
The past decade has brought promising developments. While those in medicine have been restricted to the light microscope for inspection of glass slides, whole-slide imaging (WSI) has offered a radically different medium for understanding human tissue. Whole-slide scanners create high-resolution digital images of glass slides. These scanners differ from cameras attached to or integrated with microscopes in that, while a camera captures a static snapshot of the slide of a single area at a fixed magnification, the whole slide scanner scans across the slide at every magnification (typically up to 40x), and stitches together the images into terabyte-scale files. For example, a camera attachment would produce something like the image below, while a scan would produce something like the images in this prostate adenocarcinoma biorepository*.
The implications of slide digitization in pathology are profound, and extend beyond the obvious benefits of simply migrating workflows from the lens to the monitor. Technology and research are being applied to all components of the digital workflow chain. Though currently largely fragmented and underdeveloped, such a digital workflow chain includes:
- Scanning
- Storage (and cloud migration)
- Viewing and Integration
- Computing/Processing
- Image Analysis
- Machine Learning
- Diagnostics
While scanning is peaking in its technological maturity, the infrastructure for moving, storing, accessing, and processing medical image as large as biopsy scans is still weak, to say the least. Only large hospitals and commercial pathology labs can afford the server infrastructure to manage digital pathology data. To store many terabytes of medical images requires additional IT administrators, software for management and retrieval, dozens of additional servers, redundant storage clusters, data and backup and recovery services, all of which can cost millions of dollars per year. Hospitals and commercial pathology labs are not in the business of IT, and thus require third party services that can provide cloud storage and image access systems at orders of magnitude cheaper than in-house alternatives.
Perhaps the more exciting aspect of digital pathology lies at the end of the workflow. Image analysis and machine learning techniques have been under development in research settings for the past 15-20 years. The focus of such research involves leveraging, in general, two classes of digitally extracted information to classify images as belonging to a certain category of disease state. In other words, use machine learning to diagnose. The type of information commonly extracted by computer vision and image analysis techniques are a) medically-based histological data, and b) non-parametric image features.
Non-parametric feature are general measurements that might be applied to any image (for example, an image of a dog or a boat). Measurements often involve precursory mathematical image transforms that add variance to the feature set—quantifying aspects of tissue otherwise invisible to humans. Non-parametric image features are convenient in that they are computationally efficient, algorithmically straightforward, and precise.
Histological features generally involve segmenting tissue elements (such as nuclei, stromal, or epithelial tissue) and using the shapes and relationships of such elements to construct mathematical tissue descriptions. Exemplary histological image analysis, especially for the common H&E section, has proved largely elusive. Only marginal improvements have been demonstrated over the past 5-10 years using conventional computer vision techniques. Some hope lies in both the application of machine-trained algorithms for segmentation, as well as analysis of fluorescent and immunohistochemical (IHC) stains, which are generally much simpler to quantify, though not nearly as prevalent as H&E.
Where Digital Pathology is Going
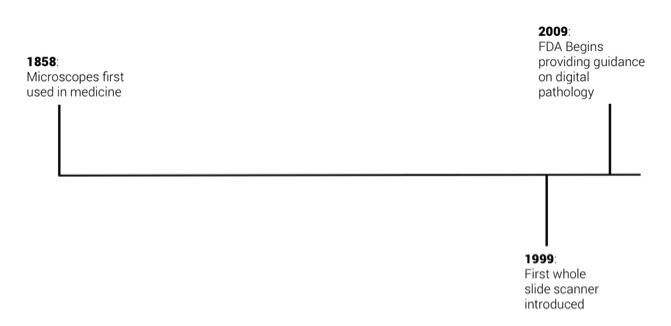
Whole slide scanners were introduced around 1999 (3), though adoption of the technology was initially slow. Digital pathology is still a new idea—the concept became widespread in medicine only over the past 3-5 years. Growth in the US over the next five years will likely be catalyzed by two key factors. The first is in FDA decision-making, and the second is in quantitative pathology service reimbursement.
FDA
In 2009, the FDA Hematology and Pathology Devices Panel convened a discussion on digital pathology. Since the discussion, the FDA has yet to provide explicit guidance on digital pathology, though is expected to soon. Such guidance will be important in that commercial products in the industry will have a specific structure for device and diagnostics approvals, as opposed to the current Band-Aide solution of applying general language to the field. Namely, the FDA has provided two documents–IVD Diagnostic products Labeled for Research Use Only and Mobile Medical Applications–that are relevant, but not specific to digital pathology (4).
Reimbursement
Currently, reimbursement for digital pathology enabled services are weak. Revisions in CTP codes by the Centers for Medicare & Medicaid Services were announce in November of this year, but were largely seen as inadequate for encouraging modern, quantitative testing which serves to benefit the patient. In December, the College of American Pathologists (CAP) put explicit pressure on Centers for Medicare & Medicaid Services to increase reimbursements on computer assisted quantification pathology services. In a recent, published letter to the organization, CAP has requested that the 2015 fee schedule be revised to include seven new pathology services, four of which would catalyze adoption of digital technologies in clinical settings. The recommended CPT codes were:
88341 – Immunohistochemistry or immunocytochemistry, per specimen; each additional single antibody stain procedure (List separately in addition to code for primary procedure) 88364, In situ hybridization (eg, FISH), per specimen; each additional single probe stain procedure (List separately in addition to code for primary procedure) 88367 – Morphometric analysis, in situ hybridization (quantitative or semi-quantitative), using computer-assisted technology, per specimen; initial single probe stain procedure 88369 – Morphometric analysis, in situ hybridization (quantitative or semi-quantitative), manual, per specimen; each additional single probe stain procedure (List separately in addition to code for primary procedure) 88373 – Morphometric analysis, in situ hybridization (quantitative or semi-quantitative), using computer-assisted technology, per specimen; each additional single probe stain procedure (List separately in addition to code for primary procedure) 88374 – Morphometric analysis, in situ hybridization (quantitative or semi-quantitative), using computer-assisted technology, per specimen; each multiplex probe stain procedure 88375 – Optical endomicroscopic image(s), interpretation and report, real-time or referred, each endoscopic session
It is likely that, while revisions may not be made to the 2015 schedule, we should expect to see new services added that will be a major stimulus for the digital pathology industry. Both FDA recognition and additional reimbursement codes are nearly inevitable, as increased strain on labs, histotechnicians, and pathologists are placing widespread pressure on the US and global tissue diagnostics infrastructure.
Overcoming those two barriers will trigger a 10-25 year wave of profound change that has not been seen in pathology for the past 150 years. Digital pathology has the potential to have the effect on medicine—particularly for diagnostics—that radiology had in the past quarter century. We will see dramatically more efficient workflows, a new era of collaboration, and more quantitative and accurate tissue assessments. Ultimately this will translate into a digitally driven leap in patient care.
References
- Silver, G. A. “Virchow, the Heroic Model in Medicine: Health Policy by Accolade.” American Journal of Public Health 77.1 (1987): 82-88.
- Huisman, Frank, and John Harley Warner. Locating Medical History: The Stories and Their Meanings. Baltimore: Johns Hopkins University Press, 2004.
- Pantanowitz, Liron, Andrewj Evans, Johnd Pfeifer, Laurac Collins, Pauln Valenstein, Keithj Kaplan, Davidc Wilbur, and Terencej Colgan. “Review of the Current State of Whole Slide Imaging in Pathology.” Journal of Pathology Informatics 2.1 (2011): 36.
- “Healthcare Regulatory Information.” Healthcare Regulatory Information. Digital Pathology Association. N.p., n.d. Web. 12 Jan. 2015.
- “CMS-1612-FC: Medicare Program; Revisions to Payment Policies under the Physician Fee Schedule, Clinical Laboratory Fee Schedule, Access to Identifiable Data for the Center for Medicare and Medicaid Innovation Models & Other Revisions to Part B for CY 2015; Final Rule; (November 13, 2014).” (n.d.): n. pag. The College of American Pathologists, 29 Dec. 2014. Web.
*The images at here are in whole or part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
Featured image taken from www.microscopyu.com
