Rewiring Pathology
for Precision Medicine
In 2003, as scientists completed the sequencing of the human genome, they painted an ambitious vision of medicine’s future. Within two decades, medicine would be transformed: designer drugs would be tailored to each patient’s genetic profile, therapy choices would be routinely guided by genetic information, and cancer treatments would target the unique signature of each tumor.1
That future is now taking shape. By targeting specific genetic mutations and proteins within a person’s biology, precision therapies are beginning to cure diseases once thought untreatable, allowing patients to live longer, healthier lives.
These advancements have been particularly impactful in the fight against cancer — a disease as diverse as it is devastating, and one that has long resisted universal solutions.
Among the early success stories, targeted therapies have achieved life-changing results in diseases like HER2-positive breast cancer and EGFR-mutated non-small cell lung cancer (NSCLC). When we can identify EGFR mutations in NSCLC patients, treatment response rates jump above 70%, compared to fewer than 30% when the biomarker is absent.2
This is a remarkable testament to the power of science and technology and their potential to benefit humanity in extraordinary ways. But we’re just scratching the surface of what’s possible. The true revolution in precision medicine — one that fully delivers on those ambitious predictions from 2003 — has yet to unfold because it has largely been missing the most fundamental window into human disease: pathology.
The missing piece in the precision medicine puzzle
Pathology has always been essential to understanding disease. Together, genomics and pathology provide a complete picture: genomics reveals what mutations are present, while pathology reveals how those mutations manifest in living tissue.
Are cancer cells aggressive? Are they responding to treatment? Is a biomarker expressed at levels that suggest a specific therapy will work? These critical questions can only be answered by studying the phenotypic manifestation of disease through tissue architecture and cellular patterns — pathology’s domain.
But today, this wealth of biological insight is completely underutilized, largely locked away in glass slides viewed through microscopes, just as they have been for over a century. This creates a stark paradox: even as we develop increasingly sophisticated precision therapies, we rely on the same basic tools to guide their use.
Digitizing pathology changes everything. By converting glass slides into high-resolution digital images at scale, we can unlock the critical data needed to power the next wave of precision therapies. AI models trained on this data can detect patterns in images that are invisible to the human eye.
These models can measure biomarkers and predict treatment response and disease progression with unmatched precision. They can even detect mutations directly from tissue images — making genomic testing more accessible and cost-effective.
Imagine rapidly analyzing the disease characteristics of thousands of patients in real time for only a few cents in computational costs. Imagine targeting EGFR mutations so precisely that almost every patient responds to first-line treatment. Imagine discovering new biomarkers for the more than 50%3 of NSCLC patients who currently lack actionable targets. Imagine nearly every patient receiving precision testing that helps their healthcare team confidently deliver the best, most personalized care.
We see this future as well within reach — if we collectively embrace the opportunity that is in front of us.
That’s why we believe unlocking the full potential of precision medicine requires rewiring pathology. In this piece, we’ll explore where we are today, the transformative opportunity ahead, and how Proscia is working to turn this vision into a reality.
Unlocking the full potential of precision medicine requires rewiring pathology.
A healthcare system at its breaking point
Despite powering 70% of downstream healthcare decisions and spending,4 pathology has stayed largely analog in a digital age. Glass slides, microscopes, and manual interpretation remain the standard. Yet, as medicine races forward, this traditional model is struggling to keep pace.
Consider this:
- Rising demand: The testing volume and diagnostic complexity continues to grow, driven by aging populations, rising cancer rates,5 and more diagnostic tests being available to patients.6
- Workforce challenges: We face a global shortage of pathologists, with fewer entering the field as demand soars.7
- Global disparities: Many regions lack sufficient pathology expertise, forcing patients to travel long distances or rely on overburdened specialists, exacerbating healthcare inequities.8

In short, outdated technology is creating a healthcare bottleneck, preventing pathology from meeting the demands of the 21st century.
These challenges have become the catalyst for a long-overdue evolution, creating momentum to finally elevate pathology into a digital and AI-driven discipline. One that not only tackles well-known operational hurdles but also unlocks lesser-known yet profound opportunities to advance precision medicine.
The digital breakthrough
Digitizing tissue in an effort to address workforce and operational challenges has already driven significant progress in several key areas of pathology:
- Accessibility: Remote collaboration and instant connection to global expertise are now possible, democratizing consultations with specialists while attracting and retaining enough pathologist talent to ensure timely case review.
- Consistency: AI-based quantitative scoring helps standardize pathologist interpretations, reducing variability variability that can negatively affect risk assessment and clinical management of disease.9
- Efficiency: Digital workflows streamline processes and prioritize urgent cases, enabling pathologists to accelerate diagnostic reviews and significantly reduce turnaround times.10
- Empowerment: AI automation frees pathologists from routine tasks so they can focus on complex interpretations that require their expertise.
But this is just the beginning. The true potential is still unfolding, with data and AI as the accelerants propelling digital pathology from its impressive foundation into a new era of transformative potential.

Left: Glass pathology slide; Right: Digitized pathology slide with multiple AI-powered assessments visualized in Concentriq, Proscia’s enterprise pathology platform.
Explosion in AI
The AI revolution has fundamentally redefined what’s possible in pathology. AI deciphers complexities within gigabytes of data, revealing hidden connections and insights previously beyond our reach. This enables translational scientists to gain a deeper, more precise understanding of disease and transform these discoveries into clinically actionable intelligence faster than ever before.
AI-powered pathology has gone from science fiction to firm reality faster than most experts predicted.
Foundation models trained on massive datasets are accelerating this transformation by extracting standardized, quantitative features from pathology images.11 Modern platforms democratize these powerful tools, enabling life sciences organizations and academic researchers — not just tech giants — to develop AI innovations and run them in scientific workflows to accelerate therapeutic breakthroughs.12
As a result, AI-powered pathology has gone from science fiction to firm reality faster than most experts predicted, unlocking capabilities at a pace that seemed impossible just a few years ago.
Even at this early stage, digital pathology and AI have demonstrated their ability to enhance drug discovery and development — from driving exploratory research13 to stratifying clinical trial patients14 and helping assess trial endpoints.15 Vision models now show exceptional accuracy in cancer detection,16 and may soon function directly as novel companion diagnostics,17 creating new opportunities for pathologists to enhance patient care and drive greater impact.
Fueled by data
These AI-driven discoveries and innovations rely on large-scale datasets that integrate multiple sources of patient information. This mirrors a broader evolution in drug discovery and development, which has moved beyond relying on single data sources — like claims, electronic health records (EHRs), and genomic data — to embrace what’s known as a “multimodal” approach. This shift reflects precision medicine’s need for a deep, nuanced understanding of human biology to precisely target the pathways driving disease.
In this new paradigm, pathology data is indispensable. Pathology images contain billions of pixels, providing one of the most direct and detailed views of disease. Two patients with the same type of cancer may have vastly different outcomes based on tissue and cellular characteristics that only pathology images can discern.
The growing adoption of digital pathology in routine practice18 is fulfilling this critical need by unlocking a wealth of real-world data that captures the full spectrum of disease variation across representative patient populations — insights that would otherwise remain trapped in analog glass slides and paper records. Unlike the carefully selected cohorts in clinical trials, this diverse real-world data reflects the true heterogeneity of disease as it exists in everyday clinical settings.
Advanced platforms with privacy-preserving technologies are pioneering solutions that combine de-identified pathology images with genomic profiles and clinical information, creating rich, longitudinal datasets. Training AI models on this combined data significantly improves their ability to predict disease progression and treatment outcomes — paving the way for powerful new tools in patient care.19
While remarkable progress has been made, like many fields, pathology is still in its infancy of realizing its full AI potential. With rapidly expanding datasets and continued technological progress, it has the power to fundamentally reshape how targeted, personalized therapies are discovered, developed, and delivered — transforming the entire continuum of care.
In the following sections, we explore key areas where digital pathology and AI are poised to revolutionize precision diagnostics and therapeutics in the near future.
The new era of AI-based treatment strategies
Advanced therapies need advanced diagnostics
The evolution of digital pathology and AI couldn’t come at a more critical time. Modern precision medicine demands advanced diagnostics to keep pace with sophisticated therapies like antibody-drug conjugates (ADCs). By combining targeted antibodies with potent cytotoxic agents, ADCs selectively attack cancer cells while sparing healthy tissue.
The potential is massive — the top three ADCs are expected to generate $17 billion in global revenue by 2028.20 But their success depends entirely on ultra-precise diagnostics to confirm sufficient target protein expression in cancer cells.
AI-driven diagnostics can bridge this gap. They can quantify biomarker expression levels, such as HER2-low levels, with exceptional accuracy and reproducibility using digital pathology images. Such subtle variations are nearly impossible to detect using traditional pathologist scoring.17,21
Even more powerfully, AI models can assess biomarker spatial distribution and per-cell expression, significantly improving drug effectiveness and patient outcomes by accounting for the “bystander effect.” This enables ADCs to target not only biomarker-expressing tumor cells but also their neighboring cells.22
By bringing this technology into everyday pathology practice, we can identify potential responders more accurately than ever before, allowing entirely new patient populations to benefit from advanced therapies.
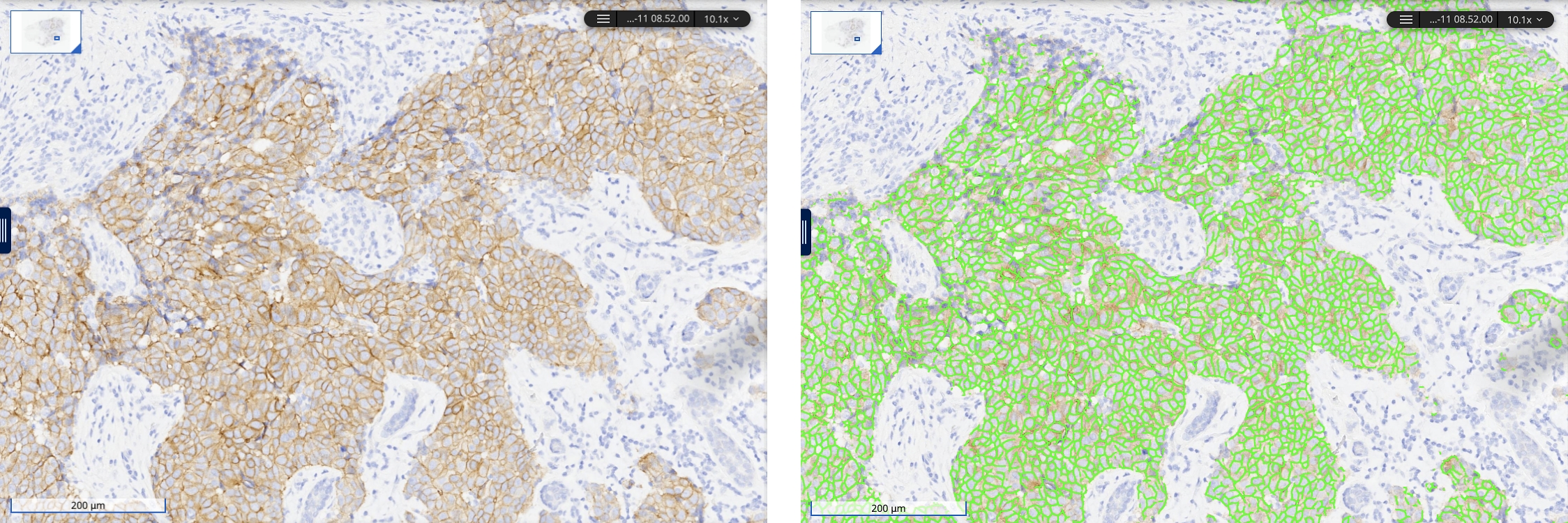
AI-based analysis of HER2 expression levels in breast cancer, viewed in Concentriq, Proscia’s enterprise pathology platform.
AI-powered patient selection: unlocking immunotherapy’s full potential
Immunotherapies like PD-L1 inhibitors have revolutionized cancer treatment, achieving long-lasting responses and even cures for previously untreatable cancers. But predicting which patients will benefit remains a serious challenge. Response rates range from 13–45%, and many patients who initially respond eventually develop resistance, leading to cancer progression or relapse.23
Digital pathology has the potential to change this by leveraging machine learning models to analyze a single image and predict response to immune checkpoint inhibitors with greater accuracy.24
Research has shown these AI-driven models can outperform current predictive methods, including tumor-infiltrating lymphocyte (TIL) density measurement and tumor mutation burden (TMB),25,26 refining patient selection and ensuring immunotherapies reach those most likely to benefit. This reduces trial-and-error treatment cycles, improves patient outcomes, and optimizes healthcare resources.
In this way, widespread clinical adoption of digital pathology could unlock the full life-saving potential of these powerful therapies already used by millions of cancer patients today.
From uncertainty to clarity: AI-guided treatment planning
Accurate disease prognosis is essential for tailoring personalized treatment plans. Digital pathology and AI can serve as powerful tools at stratifying patients into risk groups, helping clinicians provide more targeted and effective care.
Take small cell lung cancer (SCLC) — prognosis has traditionally been difficult to predict due to limited biopsy material and late-stage diagnoses. But a deep learning model using pathology images has shown remarkable accuracy in predicting outcomes.27 This breakthrough could help align treatment plans more closely with individual patient needs, ultimately improving survival rates and quality of life.
Eliminating the testing barrier to precision medicine
Today, fewer than 40% of eligible cancer patients receive essential tests like EGFR mutation screening that could match them to life-saving targeted therapies.28 While the standard genomic tests like next-generation sequencing (NGS) and polymerase chain reaction (PCR) work well, they’re woefully underutilized due to high costs, slow turnaround times, and the need for more tissue than biopsies often provide.
NGS tests frequently take an additional four weeks to return results after histopathologic diagnosis29 — an eternity for patients with aggressive disease. While newer technologies like liquid biopsies address some challenges, they come with their own limitations in sensitivity30 and accessibility31.
As the Precision Medicine Coalition puts it: “We have a widening gap between what is possible and what is practiced in modern medicine.”32
But what if we could identify patients for targeted therapies faster, using affordable, widely practiced routine clinical techniques?
We have a widening gap between what is possible and what is practiced in modern medicine.
That’s exactly what digital pathology will achieve. Genomic biomarker pre-screening can be performed directly from whole slide images, rapidly identifying patients likely to benefit from genomic tests like NGS, speeding up treatment decisions, and reducing unnecessary testing.
One study showed AI-enabled pre-screening of FGFR+ mutations from H&E-stained bladder cancer images reduced molecular testing by 30% while minimizing misclassification of patients as biomarker-negative.33
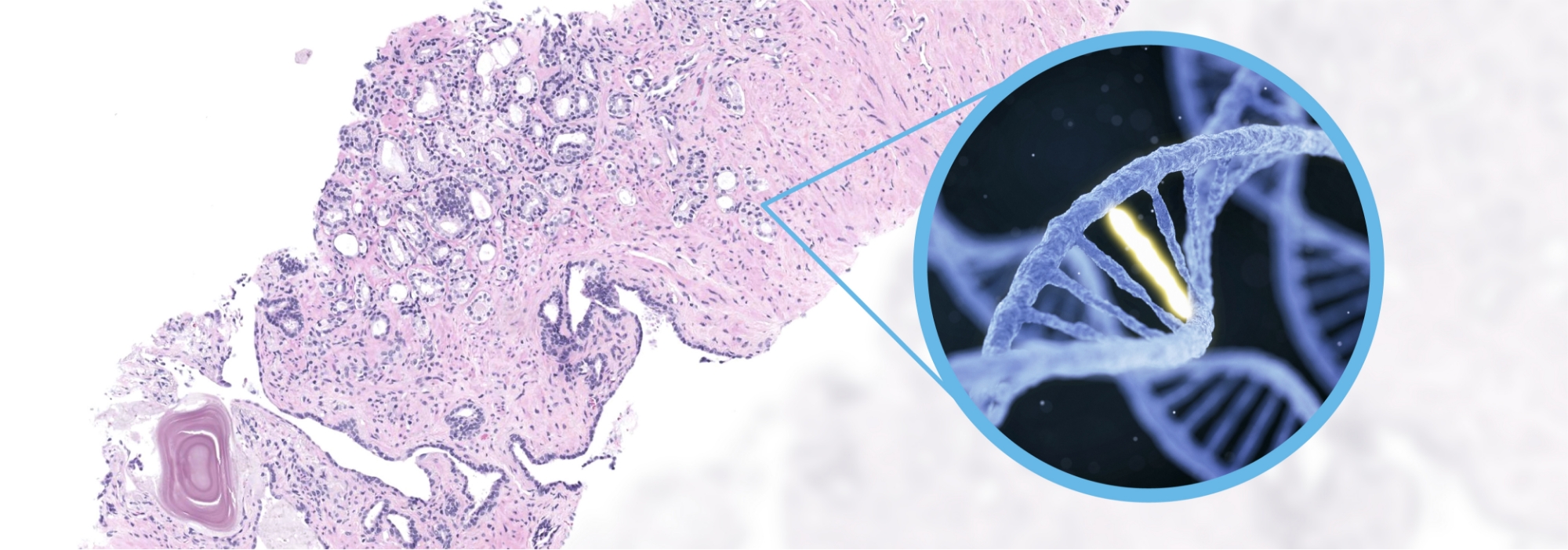
Emerging approach: Predictive biomarkers for DNA mutations using digital pathology
Implementing this approach in clinical trials and everyday patient care could revolutionize access to timely, targeted care, ensuring millions receive the treatments they need, when they need them. This is how digital pathology and AI are not just revolutionizing diagnosis and treatment — they’re democratizing precision medicine, making them more accessible to the patients who need them most.
It’s time to deliver on precision medicine’s promise
The conversation around digital pathology often focuses on two pressing challenges: the growing shortage of pathologists and the potential for AI to improve diagnostic workflows. But this barely scratches the surface of what’s at stake. The real potential is far bigger — it’s about redefining the future of medicine itself.
If pathology doesn’t modernize, therapeutic R&D remains cut off from one of the richest sources of biological insight, slowing the discovery of the next life-saving therapy. Companion diagnostics that determine whether a patient qualifies for a breakthrough treatment remain out of reach, shelving otherwise successful next-generation therapies. And the tests that should be enabling patients to benefit from precision medicine stay expensive, slow, and inaccessible to most.
When pathology is rewired with data, software, and AI at scale, we unlock a new era. One where scientists accelerate the development of next-generation therapies, patients are matched to the right treatments with unprecedented accuracy, and precision medicine becomes the standard, not the exception.
Think:
We’re closer than ever to driving this change. Proscia saw exponential growth in 2024, meeting surging demand as pathology and AI propel precision medicine forward.
We will continue to accelerate this momentum by uniting the key players and components across every area where pathology is practiced. Pathologists at renowned hospitals, health systems, and diagnostic laboratories across the globe use our platform to read cases with greater speed and accuracy while generating valuable insights for drug and diagnostic development.
Life sciences teams leverage our platform and real-world data to bring treatments to market faster and match them to the right patients through image-based biomarkers — all delivered through the same network of clinical institutions.
To make this collaborative model successful and truly valuable for everyone — life sciences, diagnostic labs, and most importantly, patients — we hold fast to core principles:
Pathologists deserve great technology
Proscia was founded to bring modern software to the people who power medicine. We recognize that our community of pathologists and scientists are on the frontlines of diagnosing disease and driving critical breakthroughs to combat cancer and other devastating conditions.
That’s why we work to earn our community’s respect by delivering outstanding performance while creating beautiful and compelling experiences that go above and beyond expectations — empowering pathologists to work at their best with confidence and efficiency day in and day out.
Harness data to advance life-saving treatments
Too often, treatments fail or disease progresses rapidly, and we don’t understand why. The answers lie in data — not in slide trays gathering dust in filing cabinets.
In partnership with diagnostic institutions, we must ensure digitized images are used to fuel the next breakthroughs in precision medicine — empowering not only scientists but also data scientists and AI teams to unlock new insights. Throughout this process, we remain committed to upholding the highest standards of patient privacy and data protection.
Propel AI forward to maximize patient impact
We have a shared responsibility to harness AI’s power to accelerate discovery and translate breakthroughs into clinical practice. We cannot sit idle and let other industries race ahead in AI innovation while patients wait for better care.
We also understand AI in healthcare requires careful consideration and brings unique challenges. But rather than intimidate us, they inspire us. We welcome each challenge as an opportunity to sharpen our focus and develop solutions that meet the highest standards, knowing that earning trust is essential to delivering AI’s benefits to patients sooner.
This commitment to excellence reflects our broader vision of collaborative innovation in pathology AI. Many talented research teams are developing intelligent AI models for diverse applications. To maximize AI’s impact and reach as many patients as possible, our platform has always been — and will always be — designed to support a growing ecosystem of best-in-class AI-powered solutions whether developed in-house, by third-parties, or our customers.
We’re also dedicated to empowering our customers — who represent some of the brightest minds in the field — with the essential data foundation and tools to drive breakthrough AI development.
With proven technology, a bold mission, and a dedicated team, Proscia is already rewiring pathology for precision medicine. But we cannot do it alone. It will take the collective effort of clinicians, scientists, technologists, drug developers, government agencies, and payers to make this vision a reality.

Time is of the essence. Patients deserve better. Science demands progress. And the future of medicine depends on it.
Sources:
1 Collins F. The future of genomics: testimony before the House Subcommittee on Energy and Commerce. National Human Genome Research Institute. Published July 22, 2003. Accessed February 17, 2025. https://www.genome.gov/11007447/2003-francis-collins-testimony-the-future-of-genomics
2 Zhang H, Zhang Y, Zhu Y, Dong T, Liu Z. Understanding the treatment response and resistance to targeted therapies in non-small cell lung cancer: clinical insights and perspectives. Front Oncol. 2024;14:1387345. Published 2024 Jul 11. doi:10.3389/fonc.2024.1387345
3 Mendenhall M, Waterhouse D, Drosick R, Ward P, Davies D. A model for achieving comprehensive biomarker testing in non–small cell lung cancer. Oncology Issues. 2024;39(1):20-25. Accessed February 17, 2025. https://www.accc-cancer.org/docs/documents/oncology-issues/articles/2024/v39-n1/v39-n1-a-model-for-achieving-comprehensive-biomarker-testing-in-non-small-cell-lung-cancer.pdf
4 Ducatman BS, Ducatman AM, Crawford JM, Laposata M, Sanfilippo F. The Value Proposition for Pathologists: A Population Health Approach. Acad Pathol. 2020;7:2374289519898857. Published 2020 Jan 14. doi:10.1177/2374289519898857
5 Sarfati D, Gurney J. Preventing cancer: the only way forward. Lancet. 2022;400(10352):540-541. doi:10.1016/S0140-6736(22)01430-1
6 Zhou Y, Tao L, Qiu J, et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct Target Ther. 2024;9(1):132. Published 2024 May 20. doi:10.1038/s41392-024-01823-2
7 Robboy SJ, Gupta S, Crawford JM, et al. The Pathologist Workforce in the United States: II. An Interactive Modeling Tool for Analyzing Future Qualitative and Quantitative Staffing Demands for Services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA
8 Levit LA, Byatt L, Lyss AP, et al. Closing the Rural Cancer Care Gap: Three Institutional Approaches. JCO Oncol Pract. 2020;16(7):422-430. doi:10.1200/OP.20.00174
9 Huang W, Randhawa R, Jain P, et al. Development and Validation of an Artificial Intelligence-Powered Platform for Prostate Cancer Grading and Quantification. JAMA Netw Open. 2021;4(11):e2132554. Published 2021 Nov 1. doi:10.1001/jamanetworkopen.2021.32554
10 Borkowski PA, Dettloff JL, Zhou J, et al. Quantifying the value of AI-powered digital workflows in real-world diagnostic laboratories: insights from a Quest Diagnostics feasibility study conducted with Proscia and Ibex Medical Analytics. Proscia and Ibex Medical Analytics; 2024. Accessed February 17, 2025. https://go.proscia.com/Quest-Study-Report-2024
11 Chen RJ, Ding T, Lu MY, et al. Towards a general-purpose foundation model for computational pathology. Nat Med. 2024;30(3):850-862. doi:10.1038/s41591-024-02857-3
12 Vorontsov E, Bozkurt A, Casson A, et al. A foundation model for clinical-grade computational pathology and rare cancers detection. Nat Med. 2024;30(10):2924-2935. doi:10.1038/s41591-024-03141-0
13 Courtiol P, Maussion C, Moarii M, et al. Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat Med. 2019;25(10):1519-1525. doi:10.1038/s41591-019-0583-3
14 Acs B, Ahmed FS, Gupta S, et al. An open source automated tumor infiltrating lymphocyte algorithm for prognosis in melanoma. Nat Commun. 2019;10(1):5440. Published 2019 Nov 29. doi:10.1038/s41467-019-13043-2
15 Frey P, Mamilos A, Minin E, et al. AI-based HER2-low IHC scoring in breast cancer across multiple sites, clones, and scanners. J Clin Oncol. 2023;41(16_suppl):516. doi:10.1200/JCO.2023.41.16_suppl.516
16 Cui M, Zhang DY. Artificial intelligence and computational pathology. Lab Invest. 2021;101(4):412-422. doi:10.1038/s41374-020-00514-0
17 AstraZeneca. Novel computational pathology-based TROP2 biomarker for datopotamab deruxtecan was predictive of clinical outcomes in patients with non-small cell lung cancer in TROPION-Lung01 Phase III trial. Published September 8, 2024. Accessed February 23, 2025. https://www.astrazeneca.com/media-centre/press-releases/2024/novel-computational-pathology-based-trop2-biomarker-for-dato-dxd-was-predictive-of-clinical-outcomes-in-patients-with-nsclc-in-tropion-lung01-phase-iii-trial.html
18 Gross DJ, Vernon LJ, DeLisle A; College of American Pathologists. 2024 Practice Characteristics Survey Report. College of American Pathologists; October 2024
19 Lipkova J, Chen RJ, Chen B, et al. Artificial intelligence for multimodal data integration in oncology. Cancer Cell. 2022;40(10):1095-1110. doi:10.1016/j.ccell.2022.09.012
20 Yang G, Smith J, Pérez L, Sartori V, Chang A, Liu L. Charting the path to patients. McKinsey & Company. January 9, 2025. Accessed February 17, 2025. https://www.mckinsey.com/industries/life-sciences/our-insights/charting-the-path-to-patients
21 Gustavson M, Haneder S, Spitzmueller A, et al. Novel approach to HER2 quantification: digital pathology coupled with AI-based image and data analysis delivers objective and quantitative HER2 expression analysis for enrichment of responders to trastuzumab deruxtecan (T-DXd; DS-8201), specifically in HER2-low patients [abstract]. In: Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium; December 8-11, 2020; San Antonio, TX. Cancer Res. 2021;81(4 Suppl):Abstract nr PD6-01
22 Kapil A, Spitzmüller A, Brieu N, et al. HER2 quantitative continuous scoring for accurate patient selection in HER2 negative trastuzumab deruxtecan treated breast cancer. Sci Rep. 2024;14(1):12129. Published 2024 May 27. doi:10.1038/s41598-024-61957-9
23 Sun JY, Zhang D, Wu S, et al. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives. Biomark Res. 2020;8:35. Published 2020 Aug 26. doi:10.1186/s40364-020-00212-5
24 Johannet P, Coudray N, Donnelly DM, et al. Using Machine Learning Algorithms to Predict Immunotherapy Response in Patients with Advanced Melanoma. Clin Cancer Res. 2021;27(1):131-140. doi:10.1158/1078-0432.CCR-20-2415
25 Hu J, Cui C, Yang W, et al. Using deep learning to predict anti-PD-1 response in melanoma and lung cancer patients from histopathology images. Transl Oncol. 2021;14(1):100921. doi:10.1016/j.tranon.2020.100921
26 Rakaee M, Tafavvoghi M, Ricciuti B, et al. Deep Learning Model for Predicting Immunotherapy Response in Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2025;11(2):109-118. doi:10.1001/jamaoncol.2024.5356
27 Zhang Y, Yang Z, Chen R, et al. Histopathology images-based deep learning prediction of prognosis and therapeutic response in small cell lung cancer. NPJ Digit Med. 2024;7(1):15. Published 2024 Jan 18. doi:10.1038/s41746-024-01003-0
28 Sadik H, Pritchard D, Keeling DM, et al. Impact of Clinical Practice Gaps on the Implementation of Personalized Medicine in Advanced Non-Small-Cell Lung Cancer. JCO Precis Oncol. 2022;6:e2200246. doi:10.1200/PO.22.00246
29 Choudhury Y, Tan MH, Shi JL, et al. Complementing Tissue Testing With Plasma Mutation Profiling Improves Therapeutic Decision-Making for Patients With Lung Cancer. Front Med (Lausanne). 2022;9:758464. Published 2022 Feb 11. doi:10.3389/fmed.2022.758464
30 Cirmena G, Dameri M, Ravera F, Fregatti P, Ballestrero A, Zoppoli G. Assessment of Circulating Nucleic Acids in Cancer: From Current Status to Future Perspectives and Potential Clinical Applications. Cancers (Basel). 2021;13(14):3460. Published 2021 Jul 10. doi:10.3390/cancers13143460
31 Habli Z, AlChamaa W, Saab R, Kadara H, Khraiche ML. Circulating Tumor Cell Detection Technologies and Clinical Utility: Challenges and Opportunities. Cancers (Basel). 2020;12(7):1930. Published 2020 Jul 17. doi:10.3390/cancers12071930
32 Abrahams E, Wells CJ. Personalized medicine: We’re not there yet. STAT. April 5, 2024
33 Juan Ramon A, Parmar C, Carrasco-Zevallos OM, et al. Development and deployment of a histopathology-based deep learning algorithm for patient prescreening in a clinical trial. Nat Commun. 2024;15(1):4690. Published 2024 Jun 1. doi:10.1038/s41467-024-49153-9