Traditional approach to peer review for non-clinical studies involving a second person review of the slide, data, and interpretation of the slide conducted by a peer has its own downsides that are eliminated by organizations that have long embraced digital pathology. For instance, a sponsor pharma company working with a CRO to conduct peer reviews using glass slides meant that either slides had to be shipped to the pathologist conducting peer review or the pathologists had to travel to the site to complete this evaluation. A digital peer review using digital pathology solution on the other hand enables organizations to streamline this process and improve efficiency.
However, ensuring compliance with the requirements to validate your digital pathology system to use in regulated non-clinical environment according to both the Code of Federal Regulations (CFR) Title 21 Part 11: Electronic Records; Electronic Signature as well as predicate rules associated with Good Laboratory Practices (GLP) documents (including 21 CFR part 58) has been a challenge for many labs as new technologies get introduced into the market. And as a digital pathology platform provider that plays an integral part of our customers’ research efforts, we know that our partnership with these customers extends beyond the technology we provide to the services we offer to help support compliance.
Proscia’s AI-powered digital pathology platform, Concentriq® for Research, is designed to provide support for GLP peer review of non-clinical (drug safety) studies. Concentriq for Research is used by leading pharma and CROs that are intricately involved in evaluating drug candidates for safety and efficacy through their preclinical laboratory studies.
This blog will highlight the Concentriq capabilities designed to meet certain validation criteria for regulated non-clinical applications, as well as highlight the services offered by the Proscia professional services team to help our customers ensure Concentriq is validated to meet these important compliance criteria.
How Concentriq for Research Can Support Your GLP Regulated Studies
Proscia’s operations are guided by an internal quality management system (QMS) to ensure our Concentriq for Research platform is GLP-ready to validate their workflows, a requirement for a GLP-ready solution. As it relates to Part 11 compliance, scientists can use Concentriq for Research for viewing purposes. Furthermore, Concentriq for Research allows users the autonomy to download project audit logs to support regulatory audits.
Configurable roles and permissions (Figure 2, below) enable users to enforce practices put in place to prevent the inappropriate deletion of raw data and to ensure that the raw data is maintained. This includes audit logs for the duration of time dictated by internal standard operating protocols (SOPs).
Let’s take a look at a few highlights of some specific GLP use cases that Concentriq for Research helps address:
- Viewing whole slides images (WSI): Setting and enforcing guidelines around image access is a fundamental component of GLP compliance and one that many labs struggle to address without a proper image management system in place. This is an area where Concentriq for Research excels. Access to WSI, whether uploaded manually or automatically, can be secured with configurable roles and permissions, along with specific permission levels. Internal or external stakeholders (such as pathologist consultations) who view WSI files in Concentriq can be restricted from modifying them and therefore cannot invalidate or alter these files. WSI files can be deleted, but that action would be captured in the audit trail, which is human-readable and exportable from the GUI.
- Viewing WSI metadata: Presenting metadata alongside WSI files is another important compliance consideration, and one that becomes more difficult as lab environments introduce technologies that may not connect seamlessly with existing systems. Similar to WSI files, the metadata associated with these images can be entered manually or imported automatically. The metadata is visible alongside the image in Concentriq for Research (Figure 1) which means that the pathologist has all the information they need to conduct their assessment inone page. The ability to modify WSI metadata can be controlled through role permissions and repository share permission levels. Additionally, WSI metadata can be viewed alongside the images.
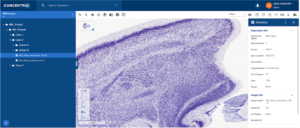
Figure 1: Metadata presented alongside WSI files.
- Data (WSI and metadata) archival: When images are no longer part of a current research project, they must be archived in a way that supports GLP compliance. Similar to viewing WSI and their associated metadata, organizations can control the access to archived data via the roles permissions and repository share permissions. Any changes to the archived data will be captured in the audit trail, which is human-readable and exportable from the GUI.
- Illustration (e.g. generating representative images for reports): Documenting representative images as part of the final study report is vital for readers and reviewers to glean insights and draw their own conclusions into the interpretations of the study being conducted. Concentriq offers the ability to take snapshots of the WSI and download as a .png. These snapshots generated within Concentriq are not stored in Concentriq unless the user manually uploads them as a case attachment. Snapshots cannot be modified in Concentriq after creation and therefore cannot be invalidated or altered. Snapshot files added to Concentriq as attachments can be deleted, but that action would be captured in the audit trail, which is human-readable and exportable from the GUI.
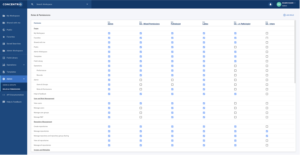
Figure 2: Configurable roles and permissions settings in Concentriq allow your organization to set more granular control for users. Categorizing permissions per role simplifies user access management based on each user’s work function.
Beyond management of images, metadata, and user permissions, data security is a vital component of GLP-compliant research operations, particularly when that data is being shared with collaborators from other organizations. Concentriq’s security standards offer end-to-end data encryption, and can scale to support the most highly-networked, high-volume digital pathology operations. As mentioned in the specific use cases above, Proscia software embraces a “private-by-default” data management design. Administrators of Proscia software have the ability to configure user roles and access permissions and grant them on an individual basis, restricting access to data to only those users who require it. Administrators also have access to audit logs that provide visibility around integrity of sensitive data.
Proscia Professional Services – GxP Validation Service
Navigating the scope of GxP validation services can be a complex undertaking while your lab is focused on the day to day research operations. Hence, we created a service offering that closely partners with your team to ensure our digital pathology solution meets compliance guidelines. Technology validation is a multi-discipline resource with intensive effort requiring extensive planning and documentation, we provide resources to our customers to help them through this process. Proscia works closely with our customers to understand their workflows and specific use cases to understand the scope of implementation. Furthermore, we prepare and offer suggestions on user acceptance testing (UAT) scripts for predefined Concentriq-based elements of your workflows. As a project management lead organization, we assign a project manager to assist you to create a plan in place to validate your system.
Accelerate Your Preclinical Research With Concentriq for Research
A digital pathology solution that can facilitate collaborative sessions between toxicopathologists of the review of assessments of trial data is necessary to maximize efficiency within your preclinical operations. Proscia’s Concentriq for Research converges digital and computational pathology to harmonize operations across the fragmented and often siloed enterprises. As a partner, we empower your organization to truly leverage the benefits of digital pathology to overcome barriers that stand in the way of advancing your research and support breakthrough innovation.
Would you like to learn more about our GLP validation services? Click here to contact our support team.
References:
Long, R. E., Smith, A., Machotka, S. V., Chlipala, E., Cann, J., Knight, B., . . . Lowe, A. (2013). Validation of Digital Pathology Systems in the Regulated Nonclinical Environment. Toxicologic Pathology, 41(1), 115-124. doi:10.1177/0192623312451162
