Pathology software can do so much more in life sciences
Biotech is moving at breakneck pace, with innovations like gene therapy, mRNA, and single-cell genomics bringing hope to patients left today without a cure. Pathology plays a vital role in life sciences, from drug discovery to development; yet, for all the cutting edge tools leveraged by pharma, biotechs, and CROs, their pathology tech stack is often stuck in 2005. Many teams touching pathology continue to rely on a combination of desktop viewers, Excel sheets, physical hard drives, S3 buckets, point-level software, microscopes, and paper notebooks. This fragmented approach leads to slow processes, lost data, and other pain points for scientists, pathologists, and leaders in life sciences.
In a16z’s blog “Doing More with Moore,” Jorge Conde and Jay Rughani touch on why. “For biotech companies, software is a means to an end—it’s science, not software, they seek to advance.” These scientists, and the organizations they work for, have been woefully underserved by Silicon Valley, but that’s changing. Pathology deserves so much more from their software.
Evolution Of Pathology Software
Pathology software is moving into its third generation, transitioning from basic viewers to image management systems (IMS) to the enterprise pathology platform.
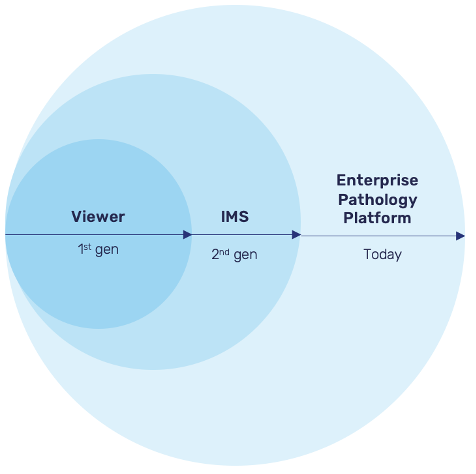
First-Generation: Viewers
Early digital pathology software emerged out of necessity, as hardware companies developed image viewers for their scanners. These desktop applications (installed at workstation), still in use in some organizations, allowed for annotation and basic metadata management but lacked more advanced capabilities.
Second-Generation: Image Management Systems
Image management systems built upon the foundation laid by viewers, combining viewing functionality with data management and collaboration capabilities. IMS became the standard in pathology software, allowing for a more centralized approach to managing images and metadata.
Third Generation: Enterprise Pathology Platform
The latest generation of pathology software goes beyond simple image management to provide a unified hub for people, data, devices, and AI applications. Enterprise pathology platforms enable organizations to better manage complex workflows and process orchestration while ensuring seamless collaboration. This category transcends the viewer and IMS in the following ways:
- Enterprise: Brings together teams across the organization onto a unified platform, allowing organizations to support complex multi-disciplinary workflows and create structured, enterprise-wide data assets accessible across multiple stages of R&D, all while preserving data security and integrity. No more point-level tools that keep data in silos. No more Excel sheets and hard drives. Organizations can count on one system of record to reflect ground truth for the enterprise. Supporting enterprise strategies helps leaders in pharma create proprietary, perennial data assets, which is critical in the age of ML / AI where the line between tech + bio is blurred.
- Pathology: More than just “managing images,” this new category of software supports richer data paradigms – marrying image data with non-image metadata, human and AI-generated, and more. With the rise in spatial biology, the convergence of molecular and imaging, and the advent of novel biomarkers that leverage multi-modal approaches, images are a central, but only partial, component of pathology data.
- Platform: The “platform” concept transcends the “management” of images to managing workflows and acting as a hub for an ecosystem of AI applications. Scientists and pathologists should be able to do their work in one unified environment. Modern APIs support the integration of image analysis tools, but also give data science and informatics teams the power to “build on the platform” by reading and writing to that enterprise system of record.
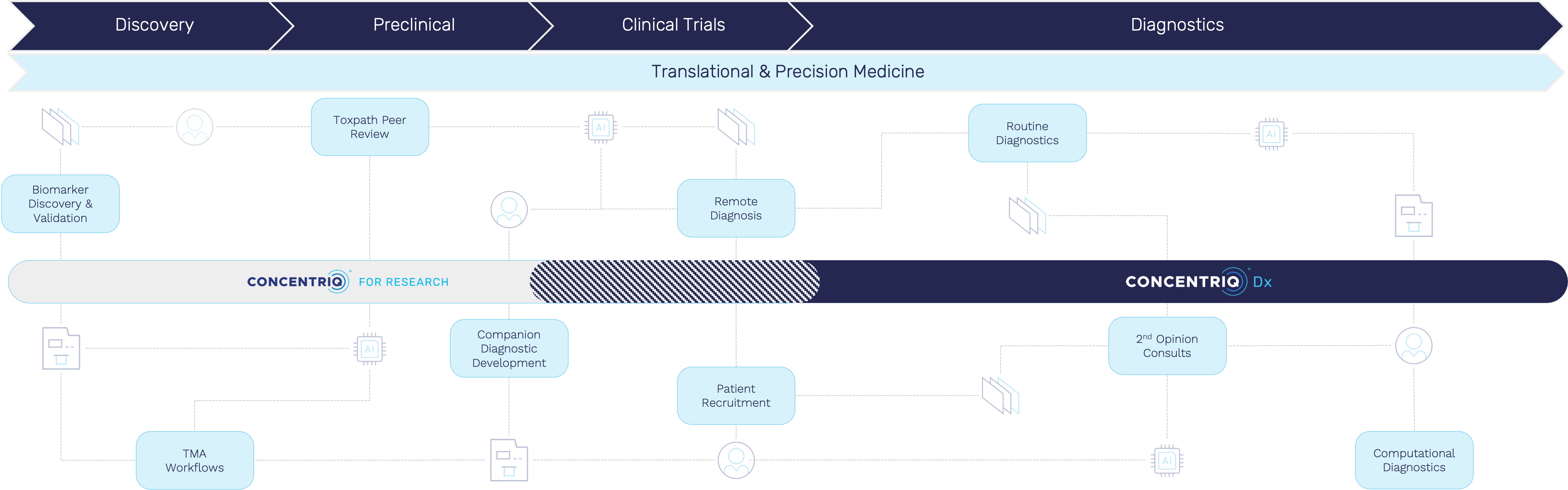
The Place Where Data Lives, And Work Happens
Our customers today look to Concentriq as their system of record for pathology – whether for a small and nimble team at an emerging biotech, or across an entire large biopharma enterprise. We pride ourselves in being the “place where pathology data lives” for our customers, and have seen them do incredible things with this. Especially at a time when AI is rapidly transforming what is possible, it’s remarkable to see how our customers are creating and leveraging complex, large-scale data sets that used to sit across Excel sheets, hard-drives, and desktop workstations.
But we think pathology software can do so much more.
Over the coming quarters Proscia will be unveiling exciting new capabilities for purpose-built workflows on Concentriq that will help streamline every aspect of working with pathology – from image ingestion to biomarker development. Pathology software should be both a place where data lives and work (the science) happens. We want to make the frustrating and tedious workflows that take weeks today, take minutes. Most importantly, we want to help our customers do their work in the same environment where the data lives, saving the laborious task of moving data between systems, and, most importantly, ensuring data integrity.
Announcing Studies
The first major update in this “place where work happens” theme is a new module called Studies. Studies takes the fundamental unit of Concentriq – the repository – and supercharges it with workflows that allow teams to work together on a unified platform while capturing new structured data from multiple stakeholders natively in the repository.
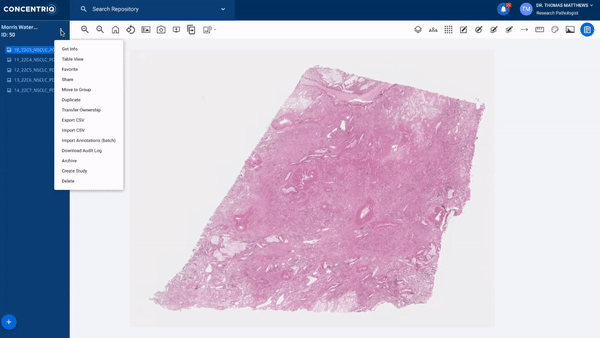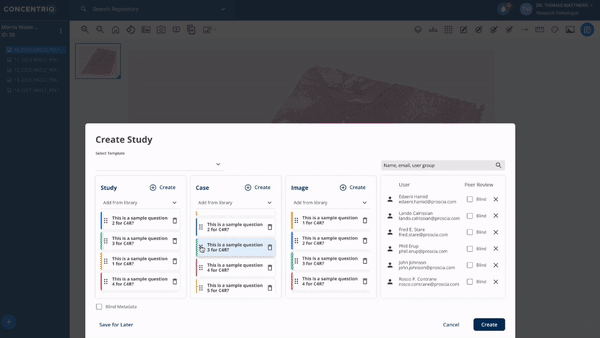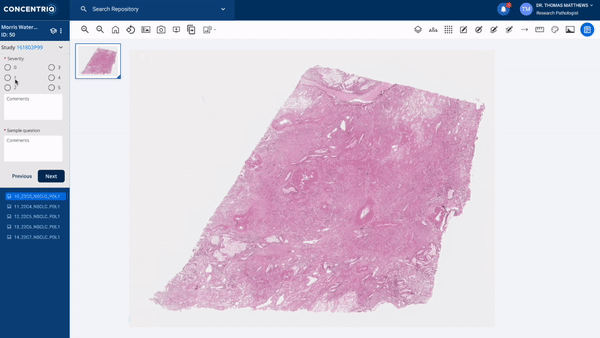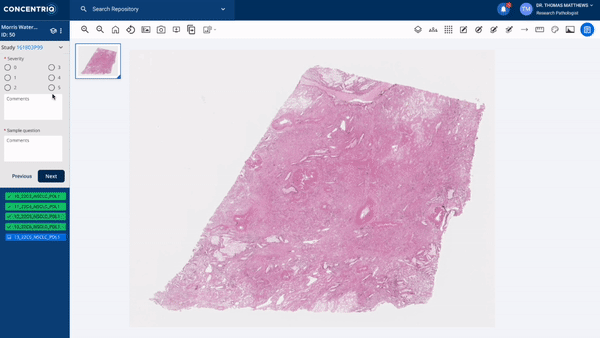
The module can help to support use cases from discovery to market, helping discovery scientists run pharmacology studies, or preclinical pathologists score images for toxicology studies, or data scientists to capture ground-truth from pathologists for biomarker development.
We announced this module this week alongside a major expansion in the toolset for GLP research and development. Studies, when married with the expanded GLP capabilities, provides preclinical teams in R&D with a powerful foundation for their work.
The best thing about Studies is that it’s still part of the same unified platform that our customers know and love. No dealing with separate workflow products – the place where the work happens is the same place as where the data lives.
I invite our customers to learn more about Studies. To give you a closer look, we’ll be hosting a webinar on May 3rd. And stay tuned for exciting new capabilities that we hope will set a new bar for what pathology software can be.
