In the challenging and lengthy process of drug development, which typically spans 10 to 15 years and costs an estimated $2.6 billion (1), a dynamic shift is occurring in the approach taken by pharmaceutical companies: quality over quantity.
AstraZeneca, for example, prioritized the quality of research and development (R&D), resulting in a notable increase in the proportion of molecules successfully progressing from the preclinical to the Phase III trial stage. The percentage has risen from 6% between 2005-10 to 19% from 2012-2016, indicating a positive shift in drug development outcomes. A pivotal element in the company’s R&D success lies in the formulation of the “5R’s Framework,” which guides teams to consider the right target, tissue, patient, safety, and commercial potential (2,3).
It should come as absolutely no surprise that the right tissue is on the list. It is imperative to collect and utilize comprehensive data to maximize the chances of success. Pathology studies play a vital role in evaluating drug-related toxicity and ensuring safety in clinical trials. The indispensability of thousands of tissue samples in investigational new drug (IND) enabling studies cannot be overlooked.
In the search for reliable contract research organization (CRO) partners, pharmaceutical companies prioritize quality pathology research. Sponsors rely on CROs with technical expertise for high-quality studies. However, during the vetting process, CROs must address important questions regarding data integrity, collaboration measures, compliance, and quality assurance. Concentriq for Research provides the ideal solution to assist CROs in addressing these critical pathology-related inquiries.
Concentriq for Research is the enterprise digital pathology platform chosen by top CROs as their central system of record. With powerful workflow and collaboration capabilities, it enables efficient and high-quality tissue analysis, even for high-volume requests. By unifying multi-site teams, data, and applications, Concentriq fosters improved collaboration and unlocks groundbreaking insights, accelerating drug discovery and development. As the only platform integrating the entire pathology ecosystem, Concentriq empowers CROs to answer difficult questions and demonstrate their expertise.
Here are a few examples challenging questions that need to be addressed to be the vendor of choice:
What specific security measures do you have in place to protect the confidentiality of pathology data, especially sensitive or proprietary information?
Protecting the confidentiality of pathology data, especially sensitive or proprietary information, is a top priority for sponsors. These questions cover topics such as data protection, compliance with privacy regulations, and the management of third-party vendors.
Proscia has achieved ISO 27001 certification after a successful third-party audit. This certification verifies that Proscia meets all requirements of the information security management standard, which establishes guidelines for people, policies, and technology to maintain an information security management system (ISMS). By choosing a software like Concentriq, CROs and sponsors alike can have added confidence in the security and confidentiality of their pathology data. It demonstrates a commitment to meeting industry standards and safeguarding data throughout the entire study process.
What measures do you have in place to ensure timely and effective communication between our teams during the course of the study?
When sponsors consider partnering with a CRO, they are particularly interested in the CRO’s collaboration approach, including information exchange and data-related matters. Sponsors want to ensure timely and effective communication between teams throughout a study and seek insights into the tools and technologies used to facilitate seamless collaboration and data sharing.
It is important to recognize that the success of a sponsor-CRO relationship is not always straightforward. Despite the sponsor organization requiring specific tests to meet scientific and regulatory standards and the CRO possessing the necessary technical expertise for high-quality studies, obstacles can arise. These obstacles often stem from a lack of clear and concise communication, as well as the need for well-defined processes that must be established and adhered to by both parties.
That’s where Concentriq, a cloud-based platform, can make a significant difference. It enables research teams from different organizations to easily exchange pathology images, annotations, and analysis results. By breaking down silos, Concentriq fosters interdisciplinary collaboration and harnesses the collective expertise of teams. It also addresses throughput and accessibility issues by providing a single click delivery of large amounts of image and study data. With automated data capture from scanners, spreadsheets, and LIMS, Concentriq enhances workflow efficiency.
Image 1 shows two scientists at work using collaboration mode in Concentriq for Research.
By addressing these concerns and leveraging a product like Concentriq, CROs can strengthen their relationships with sponsors, optimize collaboration, and accelerate breakthroughs.
Can you provide documentation or evidence of compliance with regulatory guidelines and industry standards, such as Good Laboratory Practice?
During the vetting process, sponsors often inquire about a CRO’s compliance with regulatory guidelines, such as Good Laboratory Practice (GLP). Adhering to GLP standards is crucial, as compliance is mandatory for investigational new drugs and new drug applications, making audits easier in the future.
To address these concerns, Concentriq offers a distinctive solution. It is a comprehensive platform designed to cover all of R&D – both GLP studies and non-regulated research – in a single platform. We have integrated new technical controls into Concentriq for Research to ensure compliance with GLP. Our platform provides features like audit logs, enterprise administration, and archiving workflows. Additionally, we offer GLP validation services to support teams in maximizing the benefits of digital pathology. By leveraging Concentriq, CRO leaders can confidently navigate both GLP and non-regulated studies, ensuring robust compliance and valuable insights.
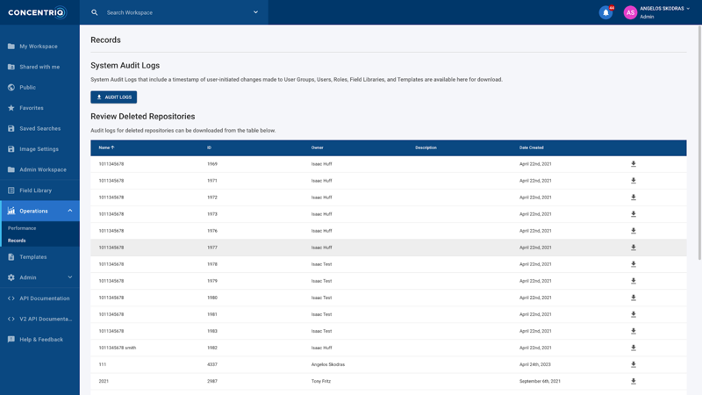
Image 2 shows an example of System Audit Logs within the Concentriq for Research platform
How do you handle quality control to minimize any potential errors that could affect the accuracy of the results?
Quality control is crucial in ensuring accurate and reliable sample collection and analysis within a CRO. Sponsors want to know that established procedures are in place to consistently meet required standards. CRO leaders should have measures in place to validate the accuracy and reliability of sample collection methods and analysis techniques. Additionally, quality control is necessary to minimize potential errors or contamination.
To optimize quality control and improve data quality, our Automated Quality Control (Auto QC) module offers an AI-enabled solution embedded in Concentriq. This module detects and segments images with artifacts, streamlining the quality control process and improving data quality for more reliable research outcomes.
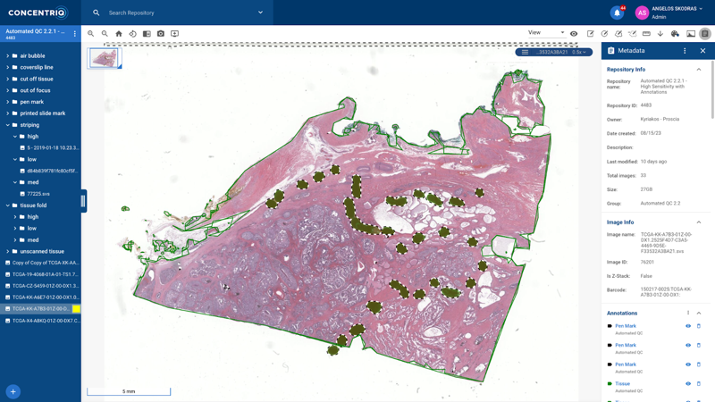
Image 3 shows an example of Automated Quality Control at work in the Concentriq for Research platform.
AutoQC sets itself apart with its vast and diverse dataset, having undergone extensive training and testing on over 70,000 whole slide images (WSIs). It ensures effectiveness and accuracy in automated quality control.
Artifact presence and batch effects in digital pathology can significantly impact downstream workflows. Automated approaches help identify slides that require reproduction or regions that should be avoided during analysis, improving the repeatability and robustness of digital pathology workflows. They also optimize workflow efficiency, reducing manual effort and time loss and preventing delays in making complete cases available for evaluation.
Prioritizing quality control procedures and implementing automated solutions, like Auto QC, optimizes quality control and improves research outcomes. CRO leaders can enhance workflow efficiency while minimizing errors and delays in pathology specimen processing by leveraging advanced technologies.
The Bottom Line
Drug developers are making a shift in focus of quality R&D over quantity of pipeline projects. Selecting the right contract research organization is essential for success. It is important for sponsors to vet potential CROs based on key areas. By addressing these key areas of concern and leveraging innovative solutions, CROs can establish themselves as reliable and trusted partners. The combination of collaboration, regulatory compliance, quality control, and data security, provided by Concentriq for Research enables CROs to drive advancements in drug discovery and development, ultimately leading to the successful bringing of drugs to market faster.
—
(1) Modernizing Drug Discovery Development and Approval. (2016, March 31). phrma.org. https://phrma.org/en/resource-center/Topics/Research-and-Development/Modernizing-Drug-Discovery-Development-and-Approval
(2) Cook, David, et al. “Lessons Learned from the Fate of AstraZeneca’s Drug Pipeline: A Five-Dimensional Framework.” Nature Reviews Drug Discovery, vol. 13, no. 6, 16 May 2014, pp. 419–431, www.nature.com/articles/nrd4309, https://doi.org/10.1038/nrd4309.
(3) Morgan, Paul, et al. “Impact of a Five-Dimensional Framework on R&D Productivity at AstraZeneca.” Nature Reviews. Drug Discovery, vol. 17, no. 3, 2018, pp. 167–181, www.ncbi.nlm.nih.gov/pubmed/29348681, https://doi.org/10.1038/nrd.2017.244
