Every diagnosis is based on data that can change the course of drug development and patient care. With the growing adoption of digital pathology, laboratories are now generating a wealth of tissue images that provide some of the most detailed and direct profiles of disease. Combined with clinical and molecular records, they offer an even fuller picture, capturing signals that are invaluable for advancing the next breakthrough therapies. Yet this rich signal has largely remained disconnected from drug development, even as biopharmaceutical companies invest heavily in precision therapies that depend on reaching the right patient populations. Enabling them to tap into it could have a profound impact. Consider that clinical trial enrollment continues to lag industry needs, and delays in Phase III alone can cost millions in lost opportunity each day.[1]
Today, we’re introducing Proscia Aperture to bridge the gap between laboratories and life sciences organizations and accelerate precision medicine. Unlike the retrospective datasets that pharma has traditionally relied on, Aperture delivers prospective, real-time insights at the moment of diagnosis. It empowers them to advance novel therapies with greater speed so that they can bring potentially life-saving treatments to more patients faster.
How Aperture Works
Aperture sits inside the diagnostic workflow, powered by Proscia’s global laboratory network on track to deliver more than 8 million pathology diagnoses this year through our Concentriq® enterprise pathology platform. Using AI, Aperture analyzes the multimodal data that converges in the laboratory, including tissue images, AI-derived biomarkers, molecular results, and clinical records. The output is evidence for biomarker prevalence studies, regulatory submissions, companion diagnostic development, label expansions, and payer negotiations. Aperture can also surface eligible candidates based on inclusion and exclusion criteria, providing the precision and representation needed to meet enrollment targets and laying the foundation for matching more patients to life-saving therapies.
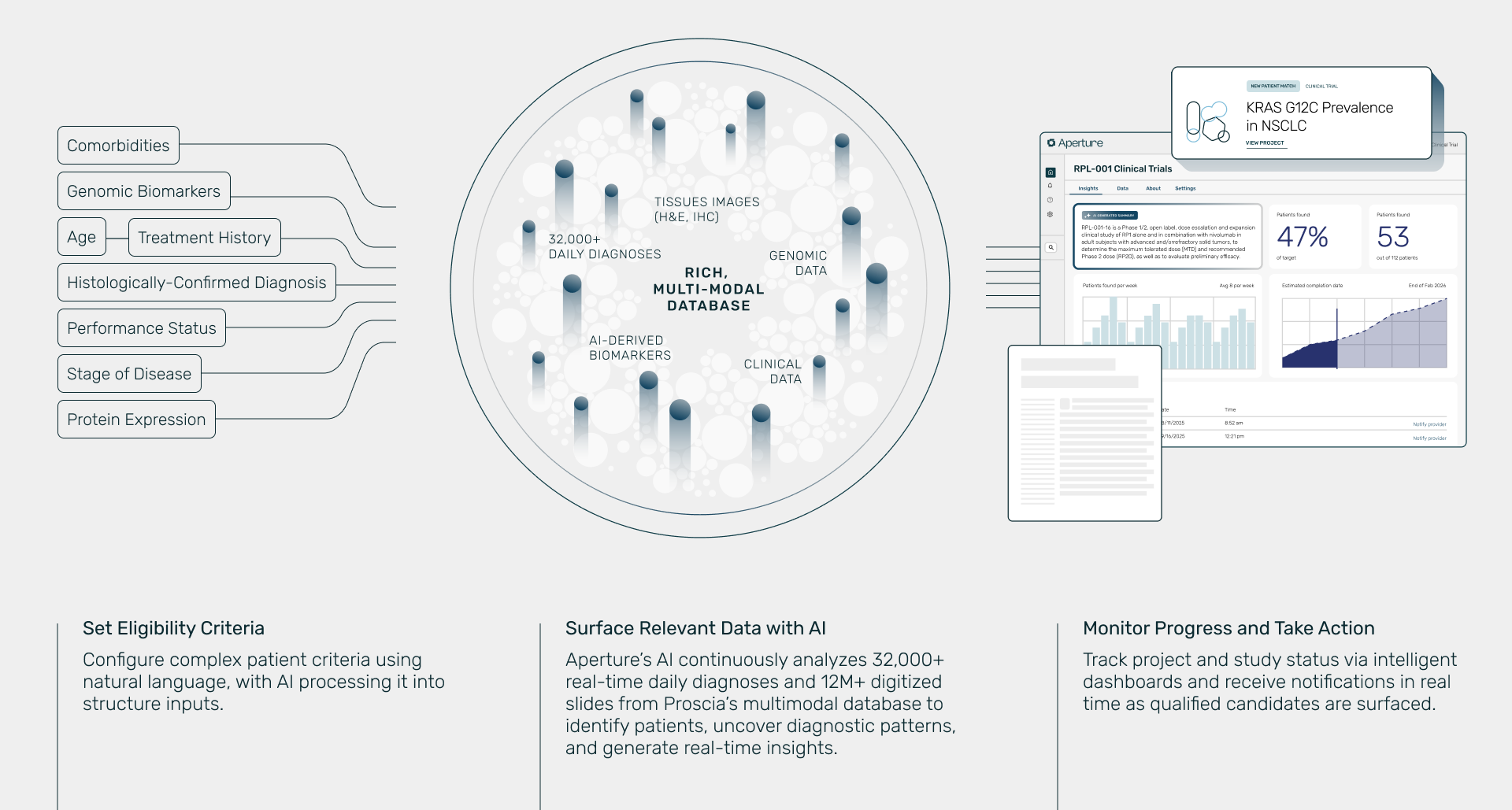
Aperture further extends its reach through Proscia’s real-world data repository of more than 12 million tissue images linked with clinical and genomic records. This retrospective data enriches the real-time diagnostic stream, ensuring that the insights delivered are both immediate and representative of the patient populations that therapies are intended to serve. All data is safeguarded through de-identification and stringent governance, so the value Aperture delivers never comes at the expense of patient privacy.
Impact in Action
Aperture creates a continuous thread across the precision medicine lifecycle. Insights that help researchers validate biomarkers and design companion diagnostics also allow clinical research teams to identify eligible patients in real time, regulators to review stronger evidence packages, and market access leaders to demonstrate value more effectively.
We’ve already seen how discoveries like TROP2, identified through pathology, have reshaped oncology by opening new therapeutic pathways. Aperture brings that type of insight into routine R&D. It also enables longitudinal datasets that are increasingly critical for regulators, similar to how real-world evidence supported the FDA’s label expansion of palbociclib (Ibrance) to male breast cancer patients, avoiding the need for an otherwise prohibitively difficult trial.[2]
For biopharmaceutical companies, the benefit is clear: fewer recruitment delays, better representation across populations, stronger submissions, and more efficient development. For patients, it means faster access to therapies that can change outcomes.
A Strategic Growth Opportunity for Diagnostic Laboratories
Laboratories have long shouldered the essential task of delivering accurate and timely diagnoses, but their role in the broader precision medicine ecosystem has often ended there. Aperture changes this. By transforming the moment of diagnosis into a gateway to life-saving therapies, Aperture positions laboratories as central partners in drug development and access.
This shift comes at a critical time, as laboratories face shrinking margins and workforce shortages that strain day-to-day operations. For those in Proscia’s global network, Aperture creates new opportunities to diversify revenue, expand services, and strengthen competitive differentiation. Most importantly, it brings these laboratories a giant step closer to patient outcomes by ensuring that insights from routine diagnoses directly inform the development and delivery of new therapies.
Accelerating the Precision Medicine Flywheel
Precision medicine is, at its core, about delivering the right treatment to the right patient. As we outline in our vision, we’ve long recognized that pathology is uniquely positioned to make this possible because of the fundamental role it plays in shaping our understanding of disease. This conviction has guided us since our earliest days, when we built Concentriq as the enterprise platform to rewire pathology from drug discovery to diagnostics.
Today, 16 of the top 20 pharmaceutical companies—those behind 34 of the 50 top-selling drugs—rely on Concentriq. Leading laboratories and health systems are on track to make 8 million diagnoses this year on the platform. In doing so, they are generating vast volumes of data-rich images that can fuel the next breakthrough therapies and diagnostics. Together, these forces create a self-reinforcing flywheel, accelerating digitization, advancing R&D, and improving patient outcomes.
Proscia Aperture is the next catalyst for this flywheel, connecting life sciences and diagnostics in a continuous cycle of intelligence. By unlocking insights at the moment of diagnosis and extending them across discovery, development, commercialization, and access, Aperture further ensures that pathology is no longer the end of the diagnostic process. Instead, it becomes the engine of what comes next, elevating pathology’s role in the precision medicine paradigm.
We’re excited to showcase Aperture at our Access25 virtual event. Register here to join us on October 28.
[1] Tufts Center for the Study of Drug Development. Quantifying the Value of a Day of Delay in Drug Development. August 2024 white paper. Tufts CSDD. Accessed via Tufts CSDD website.
[2] The Cancer Letter. “FDA uses real-world evidence to expand palbociclib label to male breast cancer patients.” The Cancer Letter, April 19, 2019. Available at: https://cancerletter.com/the-cancer-letter/20190419_2/
